Introduction
In recent years, the biopharmaceutical industry has shown a rapid growth trend around the world. This provides more opportunities for human health and disease treatment, and also puts forward higher requirements for the lighting of the production environment.
This paper expounds the requirements for space lighting, lighting fixtures and light sources from the aspects of clean environment, production technology, and material properties. This can provide guidance for lighting design and construction in the pharmaceutical industry, as well as a reference for product design and manufacturing for lighting manufacturers.
Clean Environment Requirements for Lights and Fixtures
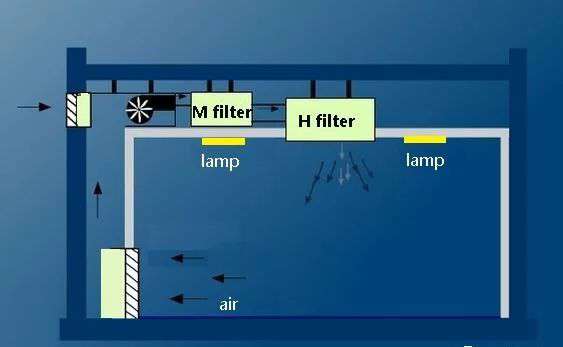
The manufacturing process of biomedicine needs to be done in a clean room, and clean room technology is based on air filtration. Different grades of clean rooms require different airflow organization methods and air filters of different specifications. Therefore, when designing clean room lighting, it is necessary to consider that the appearance and structure of lights and lanterns must meet the requirements of clean rooms.
△ Figure 1 Typical clean room structure
1.The Shape of the Light
The shape of the light should not accumulate dust. When designing, the shape of the luminaire must be designed into a streamlined structure in accordance with the requirements of fluid mechanics, without any protrusions, depressions, gaps and level differences on the surface. This is also an intuitive distinction between cleanroom luminaires and general-purpose space luminaires.
△ Figure 2 Typical clean room LED panel light
2. The Structure of the Lights
The structure of the lights cannot hinder the airflow. For this reason, the traditional lights are designed to be embedded, that is, the lights are installed in the ceiling of the clean room, but there will be many problems. Opening holes in the ceiling will not only generate high construction costs, but also reduce the mechanical strength of the roof, and leave gaps in the lights and the openings of the roof.
Where possible, the clean room lighting design should avoid embedded structures, preferably thin panel structures, and direct ceiling installation. Considering the impact on airflow, it is recommended that the thickness of the light be controlled within 15mm, or a circular arc frame structure should be used.
3. The surface of the light
The surface of the light should be smooth and uniform, and can not absorb dust, which can be satisfied by anti-static treatment. For spaces that require biological cleanliness, the surface of the light must be able to prevent bacteria, and microorganisms cannot breed after falling on the surface of the light. For this reason, the surface of the light should be made of water-repellent material, which cannot form a solution environment for microorganisms to survive.
Production Process Requirements for Lighting
The pharmaceutical production process is very complex. The production hall has numerous small-area operating rooms, as well as many changing rooms, passages and pass-through windows. These spaces are generally only a few square meters to dozens of square meters, sometimes even less than one square meter. Lighting design needs to be done while meeting power density limits.
- Illumination is the basic requirement of lighting environment. But for small spaces, the chamber type index is poor, possibly as small as 0.5 or less. The lighting manual does not even have a reference value for the space utilization factor, and the lighting design is very difficult.
- The construction cost of the clean room of the biomedical factory is very high, and it must require a high space utilization rate. At the same time, a large number of production equipment and instruments are installed in the workshop, which also affects the spatial layout of lights. For the standard requirement of 0.7 illuminance uniformity, the design needs to fully consider the installation position and light exit angle of the lights. In order to ensure high illuminance uniformity, it is necessary to combine the production process, select lights with professional light distribution, and reasonably arrange the installation positions of lights.
- Clean room workers need to work for a long time under completely closed unnatural conditions, so the requirements for light quality are higher. These requirements include control of stroboscopic and glare, and photobiological safety. For the consideration of power density, direct lighting is generally used. In architecture, it is necessary to avoid the use of highly reflective surface materials, and to use surface light sources as much as possible in the light distribution of lights, reducing the application of point light sources and line light sources.
- There are various chemical gases in the pharmaceutical production process, which puts forward explosion-proof requirements for the production space. At present, many manufacturers have launched products that can meet both cleanliness requirements and explosion-proof standards.
Requirements for the Lighting Spectrum for Pharmaceuticals and Materials
Some medicines or their raw materials have photochemical sensitivity, poor stability, and are prone to oxidation, decomposition, discoloration and other reactions when exposed to light. Especially for some chemotherapeutic drugs, complex reactions such as ring splitting, rearrangement, hydrolysis, polymerization, oxidation and isomerization can occur after dissolution. Light conditions can promote the progress of the above reactions. Therefore, these drugs need to be protected from light during production, transportation and storage. In the production process, according to the requirements of the process conditions, it is necessary to provide sufficient lighting to facilitate the production operation, and to prevent the efficacy of the medicine from being reduced by the light. In the selection of lighting equipment, high-energy light should be avoided. Generally, we recommend choosing a long-wavelength, low-energy light source.
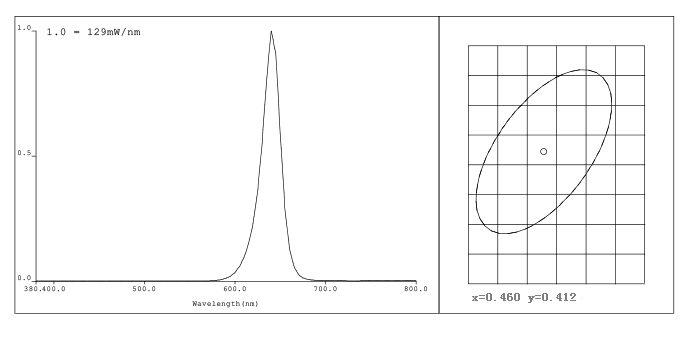
Lighting engineers and pharmacists have carried out common research on the photochemical properties of different drugs or their raw materials, selecting light sources with different wavelengths, and conducting orthogonal tests to find out the wavelengths that are the least sensitive to these drugs. Then, special lights were produced under such conditions.
Figure 3 The main wavelength of the illumination light source of calcitriol is 640nm
Conclusion
Pharmaceutical industry lighting should meet the general requirements of general lighting, but also has its own characteristics. Lighting designers should fully communicate with pharmaceutical scientists, pharmaceutical engineers, lighting and optical engineers to choose lighting products that meet process requirements. At the same time, lighting designers must cooperate with professional designers such as architecture, HVAC, and electrical to carry out lighting design while meeting clean room conditions and space requirements, and to provide a lighting environment that meets the requirements of pharmaceutical production.